Recent data suggests that 1 in 9 men are diagnosed with prostate cancer during their lifetime and one in 45 men will die from the disease. While diagnosis, management and treatment of these patients have improved over the past decade, there is much more that needs to be done. Up to 25% of patients with prostate cancer may have detectable lymph node metastases, which are correlated with a higher risk of recurrence and lower survival. Conventional imaging techniques such as CT and MRI have low sensitivity and specificity for detection of metastasis and as such are not ideal for staging of primary or recurrent prostate cancer. Pelvic lymph node dissection is considered the gold standard in assessing the presence pelvic lymph node metastasis, but its use is limited to the surgical period.
The prostate-specific membrane antigen (PSMA) receptor is over-expressed in the majority of prostate cancer and as such an accurate marker for metabolic processes and identification of metastasis. Currently, 68GA PSMA PET tracers are being used in many centers to image prostate cancer patients, but there are many disadvantages to this radionucleotide including the short half-life time and high production cost. This is of particular importance in smaller hospitals that lack the patient volume to justify an onsite 68Ge/68Ga generator.
The demand for better and more cost-effective tracers led to development of 18F PSMA. As compare to 68GA, these newly developed tracers have a longer half time, a higher positron yield, better signal to noise ratio, and better contrast resolution resulting in increased sensitivity for lesion detection. Because of the longer half-life, 18F PSMA compounds also have the advantage of being used at sites that cannot produce radiotracers due to regulatory issues and centers without access to a radionucleotide generator.
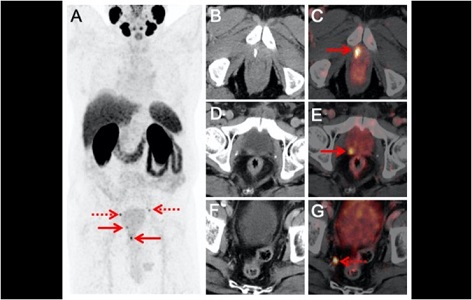
The division of Nuclear Medicine of the Department of Radiological Sciences is now participating in prospective multi-national LIGHTHOUSE Phase 3 single arm clinical trials for investigating the safety and efficacy of rhPSMA-7.3 (18F) in men with newly diagnosed prostate cancer. The principle investigator of the UCI site is Dr. Edward Uchio from the Department of Urology.
rhPSMA-7.3 (18F) is a radiohybrid PSMA-targeted receptor ligand which attaches to and is internalized by prostate cancer cells. The trial is designed to evaluate the sensitivity, specificity and positive predictive value of rhPSMA-7.3 (18F) to detect metastatic regional pelvic lymph nodes and compare the PET findings to histopathology. In addition, the study is designed to evaluate for metastatic disease in patients with negative conventional imaging.
The study will assess the impact of rhPSMA-7.3 18F PET on the staging of prostate cancer and on the clinical management of patients with biochemical recurrent prostate cancer post radical prostatectomy or post radiation therapy. rhPSMA originated from the Technical University of Munich, Germany, and has been utilized clinically for the diagnostic imaging of men with both primary and recurrent prostate cancer. In the future, rhPSMA compounds will also be labeled with radioisotopes such as 177Lu and 225Ac for therapeutic use.
The division of NM is happy to take part in this multicenter study that evaluates the safety and efficacy of rhPSMA-7.3 (18F), a highly innovative PET tracer that has the potential to significantly enhance the clinical outcome of patients with prostate cancer.
Tatiana Kain, MD

