Less invasive approach helps treat patients with renal tumors.
There are over 50,000 new cases of RCC diagnosed each year in the United States. Many of these masses are detected on routine imaging performed for other reasons. There has been increasing interest in treatment of these small lesions due to advancements in percutaneous ablation techniques. Cryoablation has emerged as a viable alternative to surgical techniques like partial nephrectomy in many cases. Preservation of normal renal parenchyma is an advantage of cryoablation. In addition, these minimally-invasive ablations can be performed on patients who are not surgical candidates. In general, there are fewer serious complications and morbidity as compared to surgery while maintaining favorable outcomes.
The ideal treatment candidate would have a small RCC less than 4 cm in diameter that is confined to the kidney. Lesions near the hilum or the central collecting system are not ideal for a percutaneous approach due to the difficulty in placing the ablation probes safely and achieving a negative margin. Other potential patients are those that have a solitary kidney or those with syndromes like Von Hippel-Lindau. These patients are often ideal for cryoablation because of the ability to preserve as much normal renal parenchyma as possible. Sometimes larger lesions can be treated if a patient is a poor surgical candidate due to age or other comorbidities. However, tumors greater than 4 cm can result in post ablation hemorrhage. New techniques involving pre- ablation embolization followed by cryoablation in the same setting are being performed by Interventional Radiologists with good results.
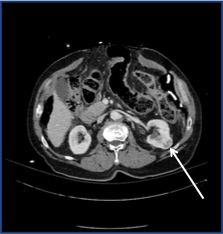
The images below show a typical case. This patient was discovered to have an incidental 3 cm left renal mass on imaging. The intraprocedural CT clearly shows the placement of the cryoablation probe as well as the low-density ice ball surrounding the lesion.
The cryoablation itself is usually carried out as an outpatient procedure. The cryoablation probes are placed with ultrasound and CT guidance. Once the probes are in place, 2 freeze-thaw cycles are performed. The repeated freezing and thawing disrupts the cell membrane and leads to cell death. An advantage of cryoablation is that the “iceball” can be visualized with non contrast CT during the procedure in order to monitor the ablation zone. The probes are then removed and a small bandage is applied to the puncture site. Most patients are able to go home 4 hours after the procedure and are back to normal activity within a couple of days.
Cryoablation is currently offered by our subspecialized interventional radiologists at UC Irvine.
James Katrivesis, MD